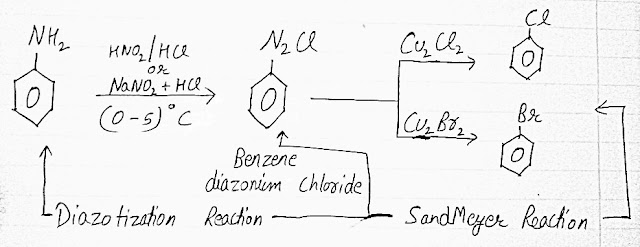
SANDMEYER REACTION
- The DIAZONIUM (C6H5N2Cl) is prepared by treating ice cold solution of ANILINE (C6H5NH2) in excess of dilute HCl (Hydrochloric acid) with an aqueous solution of NaNO2 at low temperature (0-5)°C and the reaction is known as DIAZOTIZATION REACTION.
- BROMO and CHLORO ALKANES can be prepared by treating a freshly prepared DIAZONIUM SALT with CUPPEROUS BROMIDE or CUPPEROUS CHLORIDE and this reaction is called as SANDMEYER REACTION.








6 Comments
it shouldn,t be haloalkanes.if you add a halogen in aromatic compound
ReplyDeleteit will form haloarene or aryl halide.
u r right
Deletethanks for help
ReplyDeletegr8 for last min revisions
ReplyDeleteThanks for this well-researched article—it gave a clear explanation. Read this article completely for full details Online Games. CPS Games is a fun and easy-to-use browser gaming platform.
ReplyDeleteI’m thankful for this information-packed article—it helped me a lot. Read this article for complete explanation Online Games. CPS Games offers a wide range of simple games.
ReplyDelete